Word Templates
![]()
All eCTD dossiers start their life as a set of individual documents, authored around common structures and containing standard information. Creating these documents can be a time-consuming process involving many different authors, working with numerous different documents. With each individual author writing in their own style, the resulting documents are often inconsistent in appearance and format. This often leads to a lengthy review, both internally and at the agencies.
EXTEDO eCTDtemplates provide a simple way to create consistent and compliant electronic Common Technical Document (eCTD) dossiers. Through their library of over 1300 pre-written MS Word templates, eCTDtemplates deliver a common starting point for all your technical documentation. By ensuring consistent authoring standards, eCTDtemplates enable authors to focus on content and automate the style. This approach reduces common errors and provides a harmonized structure for non-regulatory personnel.
Being created to focus on global guidance and format standards, such as those issued by the ICH and adopted by the US FDA and the EMA, eCTDtemplates ensure that your documents contain all the sections and instructions you need. As the regulations are routinely updated, eCTDtemplates are also maintained continuously to support regional variations.
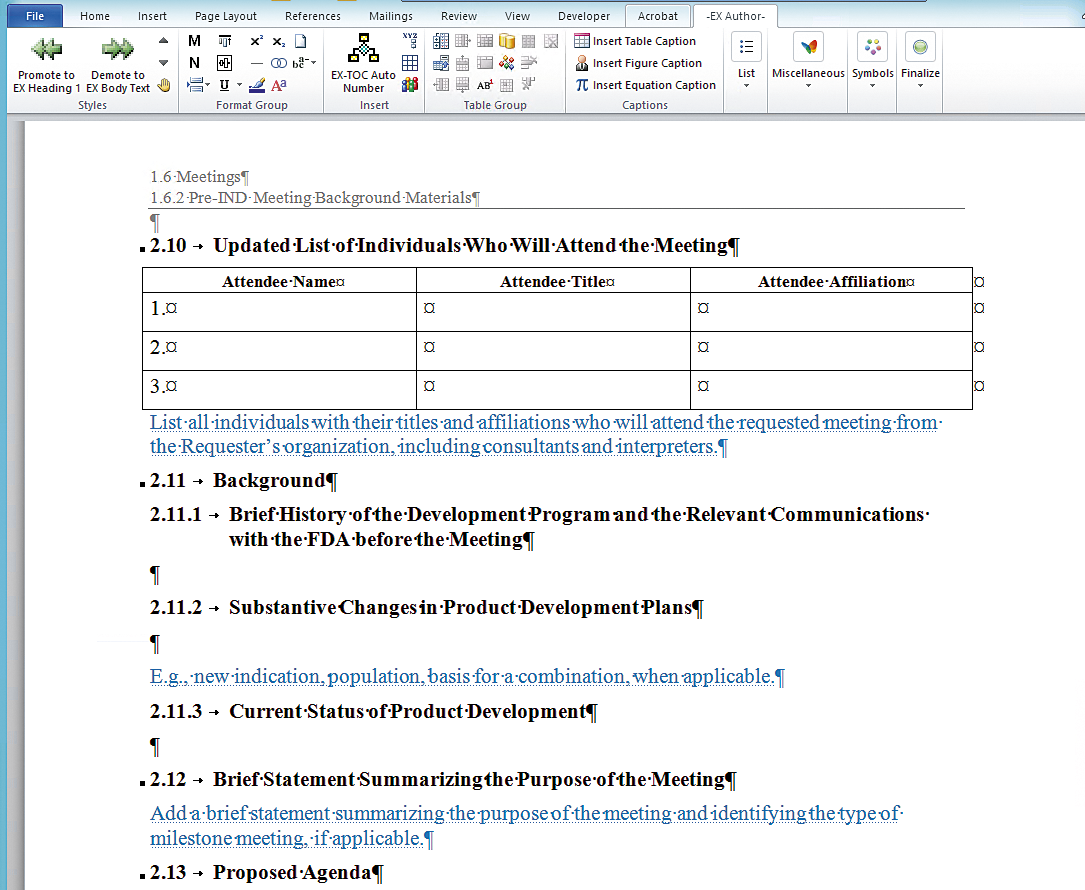
Reduce re-work and speed up approvals
Inconsistent writing styles and non-compliant information within documentation can lead to time consuming updates and re-writes. At best, this could result in approvals being delayed by the authorities or worse, it can result in agencies refusing to accept files during their technical assessment.
Through the provision of inline guidance text for authors, eCTDtemplates indicate what information needs to be entered into each individual area for each document type. eCTDtemplates also ensure that your information is structured consistently and accurately entered. This enables reviewers to quickly locate the information they need and make process reviews and approvals faster.
Speed up the authoring process for both Medical Writing and Regulatory Affairs
eCTDtemplates include the EXTEDO Word Toolbar to assist your authors in creating their content. The EXTEDO Word Toolbar contains 60 essential buttons to reduce the time needed to perform the most common authoring tasks in a single click. It can automatically create a Table of Content, Table of Figures or Table of Tables; add footnotes to a table; and remove your hidden text.
The EXTEDO Word Toolbar also makes it easier for regulatory departments to make documents submission-ready. When authors use the toolbar to create cross-references to other sections, these cross-references can be automatically created as hyperlinks when the document is converted to PDF. Similarly, the use of heading styles allows for the automatic creation of bookmarks. Using the EXTEDO Word Toolbar enables regulatory publishing to be complete faster and with less re-work.
eCTDtemplates provide Effortless Compliance™ with industry best practice and writing guidelines for eCTD documentation. They improve your documentation standards, minimize re-work and optimize time critical document production processes.
点击下载产品彩页
上一篇:RLPmanager
下一篇:eCTDmanager


